Malaria this month, sexual exploitation, sepsis, prolonged jaundice and DDH. Do leave comments below:
Diagnosing malaria in children
In children with suspected malaria do we need three negative blood films to exclude a diagnosis of Malaria?
– with thanks to Dr Tom Waterfield for summarising his recent article (5) below for Paediatric Pearls
There are over 300 new cases of imported paediatric malaria in the UK each year and cases of imported malaria here have been increasing over the last 20 years (1). Malaria in children is particularly difficult to diagnose because the initial presenting features are subtler than in adults. Children may appear quite well initially with a fever and no focus but; they are at risk of a rapid deterioration and are more likely to develop severe malaria.
The “gold standard” for ruling out the diagnosis of malaria if clinically suspected is three negative thin and thick blood films (2). This approach however, relies on serial phlebotomy and the availability of adequately trained staff. Furthermore, during out-of-hours periods the time and resources required are likely to result in delays in obtaining results especially if trained staff have to come in from home. There are now a range of Rapid Diagnostic Tests (RDTs) that are highly specific and sensitive for malaria. So are three films really required when we have RDTs?
There is only one study exploring the combination of blood films together with RDT’s in diagnosing imported malaria and it was in adults (3). Of the 388 cases, 367 (95%) were diagnosed by the initial blood film. Of the 21 that weren’t diagnosed on the blood film 19 had RDT’s performed. This diagnosed a further 10 leaving only 9 cases (2.3%) not picked up by a single blood film and RDT. Only one case of P.falciparum infection was missed and this was in a partially immune individual who had already received an unspecified treatment. The remaining 8 missed cases were P.vivax and P.ovale.
If we extrapolate from this study, then if a single blood film and RDT are negative a diagnosis of malaria is extremely unlikely. This is especially true in cases of suspected P.falciparum in a non-immune patient who has not received any treatment. The most obvious criticism here, is that it is difficult to extrapolate adult data and draw conclusions relating to children. However, the available data comparing parasite counts between children and adults suggests that on average children have a comparable or higher parasite count than adults (4). This would suggest that the results seen for adults would be comparable or even favourable in children.
Because of the paucity of data overall and lack of paediatric data it is not possible to make a blanket recommendation. The risk of malaria in each individual needs to be considered in conjunction with investigation results. For more information on diagnosing malaria in children read – How to interpret malaria tests (5).
References:
1. Ladhani S, Garbash M, Whitty CJ, Chiodini PL, Aibara RJ, Riordan FA, et al. Prospective, national clinical and epidemiologic study on imported childhood malaria in the United Kingdom and the Republic of Ireland. Pediatr Infect Dis J. 2010;29(5):434-8.
2. England PH. THE PHE MALARIA REFERENCE LABORATORYLABORATORY USER HANDBOOK. Public Health England2015.
3. Pasricha JM, Juneja S, Manitta J, Whitehead S, Maxwell E, Goh WK, et al. Is serial testing required to diagnose imported malaria in the era of rapid diagnostic tests? Am J Trop Med Hyg. 2013;88(1):20-3.
4. Mascarello M, Allegranzi B, Angheben A, Anselmi M, Concia E, Lagana S, et al. Imported malaria in adults and children: epidemiological and clinical characteristics of 380 consecutive cases observed in Verona, Italy. J Travel Med. 2008;15(4):229-36.
5. Dyer E, Waterfield T, Eisenhut M. How to interpret malaria tests. Arch Dis Child Educ Pract Ed. 2016 Apr;101(2):96-101. doi: 10.1136/archdischild-2015-309048. Epub 2016 Feb 2.
June 2016 published
Curly toes this month to herald the start of a new series on paediatric orthopaedics, sexual bullying, jaundice in the neonatal period and periorbital cellulitis. Do leave comments below…
Periorbital and orbital cellulitis in children
Peri-Orbital and Orbital Cellulitis in Children
With thanks to Dr Kat Smith, paediatric registrar and education fellow at King’s College Hospital….
The somewhat red, somewhat swollen eye is a relatively common presentation in children, and distinguishing between peri-orbital and orbital cellulitis hinges closely on an examination which can be difficult to perform in young children who cannot communicate pain on eye movement or subtle changes in vision.
Back to basics
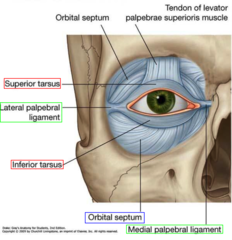
(Diagram above from quizlet.com)
The orbital septum is key in differentiating between peri-orbital and orbital cellulitis, and in dictating management. For those of us who haven’t thought about it since medical school, it is an extension of the periosteum of the frontal plate of the upper eyelid; a tough structure, where infection cannot pass from front to back unless the septum is breached by a sharp object. However, the orbital septum is not as thick and well developed in infants as it is in older children and adults, and so is not as effective a physical barrier in this age group.
Peri-orbital (or pre-septal) cellulitis is inflammation and infection of the eyelid soft tissue superficial and anterior to the orbital septum; the septum itself is not affected. Ocular function remains intact.
Orbital (or post-septal) cellulitis is infection of muscles and fat within the orbit, posterior to the orbital septum; the septum itself can be affected. It’s location in muscles and fat leads to associated ocular dysfunction.
What’s different in children?
Children are twice as likely to develop periorbital and orbital cellulitis in comparison to adults, and whilst in adults peri-orbital cellulitis is usually secondary to a superficial injury, children may develop it secondary to an occult underlying bacterial sinusitis (in particular, through the thin and porous ethmoid bone; there is often a history of recent URTI) or due to spread from another primary infection, such as pneumonia.
This difference in underlying aetiology means that in children a peri-orbital infection can rapidly progress to the much more concerning condition of orbital cellulitis, with the associated risk of rare but serious complications such as abscess formation, cavernous sinus thrombosis, intracranial abscess, and loss of vision.
Examination
The happy, well-looking child who is able to open their eye sufficiently for you to demonstrate normal light reflexes and see that they comfortably move their eyes in all planes more than likely has peri-orbital cellulitis; this will be most children. However, there are red flags that make orbital cellulitis a likely diagnosis and should prompt urgent referral to secondary care:
– Eyelid swelling such that the eye is not visible
– Toxic / systemically unwell
– CNS signs or symptoms
– Severe / persistent headache
– Pain on pressing the closed eyelid, indicating septal involvement
– Pain on eye movement, indicating involvement of muscle and / or fat
– Diplopia; older children should be able to describe “seeing double”, younger children may become unsteady when walking or struggle to grab objects
– Reduced visual acuity; the younger child may struggle to play with smaller / more “fiddly” toys
– Proptosis
– Ophthalmoplegia
– Absent light reflexes
– No improvement or worsening despite 48hrs oral antibiotics
– Neonatal age group (may be congenital dacryocystitis)
Management
Most children will be well, with mild-moderate swelling and erythema and no red flags; these children can initially be managed in the community, and most will not require later referral to secondary care.
Children with mild-moderate eyelid swelling, no significant erythema and an obvious cause – such as a chalazion or insect bite – do not have peri-orbital cellulitis, although they may need advice or treatment for the underlying cause such as warm compresses or anti-histamines.
Those with mild to moderate swelling, erythema and no obvious cause but no red flags are likely to have peri-orbital cellulitis and so require oral antibiotics; typically a 5-7 day course of co-amoxiclav is given, although this varies dependent on local microbiology guidance. Because of the underlying aetiology of peri-orbital cellulitis in children, parents should be advised that if children develop any red flag symptoms they require immediate medical review, and a GP review should be arranged for 48 hours’ time to ensure that symptoms have started to improve.
It can be unclear in young children if they have any red flags; if in doubt, refer to secondary care for review by ophthalmology, A&E, or paediatric teams. Even in secondary care it can be unclear, and children may be admitted simply for oral antibiotics and observation. ENT teams will also need to be involved if orbital cellulitis is suspected.
As above, children with any red flags are likely to have orbital cellulitis and will likely require admission to hospital for blood tests, cultures and IV antibiotics +/- imaging of the sinuses and orbits (although more extensive neuroimaging is indicated if there is a suspicion of cerebral infection).
References
“Children are twice as likely to develop periorbital and orbital cellulitis in comparison to adults”
Robinson A, Beech T, McDermott A, et al. Investigation and Management of adult periorbital or orbital cellulitis. J Laryngol Oto. 2007;121:545-7.
Bibliography:
BMJ Best Practice: Peri-orbital and orbital cellulitis. Available from http://bestpractice.bmj.com/best-practice/monograph/734.html
Clarke W. Periorbital and orbital cellulitis in children. Paediatr Child Health. 2004;9(7):471-2
The College of Optometrists. Clinical Management Guidelines. Cellulitis, preseptal and orbital. Available from http://www.college-optometrists.org/en/utilities/document-summary.cfm/docid/25FDE60B-E41D-4212-8AB02819A83E72E1
May 2016 PDF uploaded for the bank holiday
Reintroduction of egg this month with thanks to the BSACI, benign acute childhood myositis, NICE on iv fluids plus a couple of links on when not to use this guidance and a comparison of algorithms for children with a non-blanching rash. Do leave comments below.
April 2016 PDF digest
April 2016’s offering ripe for reading over the bank holiday weekend. Last text box from the 2014 BTS asthma guideline – this time on acute management, FGM and the importance of reporting colleagues who may be involved in the practice, Group A strep infection as a complication of chicken pox and some links to some good CPD sites for you and your patients.
We also welcome Dr Kat Smith this month, paediatric registrar and education fellow at King’s College Hospital, who has kindly volunteered to write monthly articles for the newsletter. It’s nice to have a fresh pair of eyes on paediatric topics and a fresh nose to the ground so to speak. Thanks, Kat, for your help.
Do leave comments below.
Invasive Group A Strep (GAS) and chicken pox
With thanks to Dr Kat Smith, education fellow and paediatric registrar at King’s College Hospital who answered my call last month for more writers to help me put together the monthly Paediatric Pearls newsletters.
Group A Streptococcal Infection in Chickenpox
Chickenpox in children is common and usually follows a mild and self-limiting (if somewhat itchy) course. After an incubation period of 10-21 days the first signs of illness are viral prodrome, mild pyrexia, and the classic cropping vesicular rash; the pyrexia is typically mild (38-39oC) and lasts 3-4 days.
In otherwise healthy children the most common complication of chickenpox is secondary bacterial skin infection, typically caused by scratching lesions. Whilst most of these are mild impetigo or localised cellulitis, the increased incidence of group A streptococcal (GAS) colonisation in children (around 10% are asymptomatic carriers in the throat or on skin) makes invasive GAS infection a real concern.
Secondary bacterial skin infection
This is characterised by erythema +/- tenderness around lesions. Children may be well in themselves if the infection is superficial; if they become more unwell this raises the suspicion of a more serious or invasive bacterial infection.
Serious bacterial superinfection / Invasive GAS infection
Around a third of children admitted to hospital with chickenpox have secondary skin infection, some of whom develop invasive infections such as pneumonia, osteomyelitis and septicaemia. GAS in particular can be associated with more fulminant infectious processes such as necrotising fasciitis and toxic shock syndrome (TSS); both are associated with high mortality and morbidity in children.
Features that should prompt consideration of a serious bacterial superinfection are:
- A lethargic or unwell-looking child; remember, children with chickenpox are typically uncomfortable but well.
- Spiking, high-grade pyrexia
- Pyrexia for longer than 4 days, particularly after initial improvement
- Diarrhoea or vomiting
- Soft tissue pain which seems disproportionate to other examination findings (an early sign of necrotising fasciitis)
How to prevent bacterial superinfection
Because scratching lesions is the most likely way to allow bacteria to breach the body’s normal defences, the primary aim of prevention is to limit scratching:
- Keep skin moisturised. Many parents still use calamine lotion but it is worth noting that it becomes ineffective once dry, and traditional emollients (e.g. 50:50) may be more effective.
- There is evidence that sedating antihistamines offer some benefit; chlorphenamine is licensed for this use.
- Dress children in smooth, loose, cotton clothing.
- Keep fingernails trimmed short.
- There are rare reports of NSAIDs potentially worsening skin infections in chickenpox, so ibuprofen should be used with caution. In practice, it would be unusual for a child to need ibuprofen if receiving regular paracetamol; pain or pyrexia necessitating its use in addition to paracetamol should prompt consideration of serious bacterial superinfection.
- There is no evidence to support the use of acyclovir in young, immunocompetent children with self-limiting, uncomplicated chickenpox; it does not decrease the incidence of complications.
What to do if you suspect bacterial superinfection
- Otherwise well children with evidence of few, small areas of bacterial superinfection can be managed in the community with oral antibiotics and safety-netting advice.
- Children with evidence of collection, extensive areas of bacterial superinfection, who are unwell, or have other features consistent with possible serious bacterial superinfection, need urgent referral to secondary care.
- In secondary care, unwell children with evidence of shock / sepsis need urgent resuscitation and intravenous antibiotic administration; if possible this should include clindamycin, due to its vital role in inhibiting toxin production by GAS.
- Invasive GAS infection has high mortality, and if suspected there should be a low threshold to involve senior staff, regional PICU services, and in the case of necrotising fasciitis, surgical teams (for early debridement); early use of inotropes and IVIG may also be required.
Bibliography
Chickenpox NICE Clinical Knowledge Summary (which I found to be the best resource by far): http://cks.nice.org.uk/chickenpox
Cohen J, Breuer J. Chickenpox treatment. Systematic review 912. BMJ Clinical Evidence.
Papadopoulos, AJ. Chickenpox. emedicineWebMD. www.emedicine.com
References
Re: “the increased incidence of group A streptococcal (GAS) colonisation in children (around 10% are asymptomatic carriers in the throat or on skin)”
Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010 Sep;126(3):e557-64
Re. “Around a third of children admitted to hospital with chickenpox have secondary skin infection”:
Bovill B, Bannister B. Review of 26 years/ hospital admission for chickenpox in North London. Journal of Infection. 1998;36(suppl1);17-23.
Re: “necrotising fasciitis and toxic shock syndrome (TSS); both are associated with high mortality and morbidity in children.” AND “IVIG may also be required.”
Chuang YY, Huang YC, Lin TY. Toxic shock syndrome in children: epidemiology, pathogenesis, and management. Paediatr Drugs. 2005;7(1):11-25.
Re. “There is evidence that sedating antihistamines offer some benefit”
Tebruegge M, Kuruvilla M, Margarson I. Does the use of calamine or antihistamine provide symptomatic relief from pruritus in children with varicella zoster infection? Archives of Disease in Childhood. 2006:91(12);1035-1036.
Re: (continued from above) “chlorphenamine is licensed for this use.”
BNFC, available at: https://www.medicinescomplete.com/mc/bnfc/current/PHP1934-chlorphenamine-maleate.htm?q=chlorphenamine&t=search&ss=text&tot=40&p=1#_hit
Re: “if possible this should include clindamycin, due to its vital role in inhibiting toxin production by GAS.” (as well as having it drilled in to us by the microbiologists at St Thomas’):
http://emedicine.medscape.com/article/228936-medication#2
March 2016 uploaded
March 2016: a few odds and ends on asthma this month and assessing a child in an acute exacerbation, Childline survey, Meningococcus W and paediatric neck lumps. Do leave comments below:
February 2016
Stepwise management of asthma this month. Plus some information on infant mental health, paediatric airways and a few more sites on internet safety. Do leave comments below.
Parent Infant Mental Health
With thanks to Geoff Ferguson, Director of the Parent Infant Centre (www.infantmentalhealth.com) for the following explanation of the Acquarone scales:
The Acquarone Detection Scales for Early Relationships are observational scales that provide a powerful tool for assessing an infant’s capacity to form relationships and a mother’s ability to respond to her infant. The scales have been developed during several decades of clinical practice by Dr Stella Acquarone, who is also the author of several books on infant development and parent infant psychotherapy and Principal of the Parent Infant Clinic. The Parent Infant Clinic is a private service but does have some subsidised places for families with limited financial resources.
There are two scales, a 25 item scale for observations of the infant and a 13 item scale for observations of the mother. In each case observations are divided into four domains: interpersonal, sensorial, motor and affect. Within each domain observers are asked to note the frequency of certain behaviours. For example, when observing ‘calling’ the observer is looking for ‘facial expressions, noises or gestures that seek to produce an affectionate response from the partner’.
A concern about the infant or the mother might be raised if a particular behaviour was never observed, perhaps showing a difficulty in relating, or was constantly observed, perhaps showing a defensive repetitiveness. The scales can be used to establish a thorough observational benchmark against which later changes can be compared.
Click here to see an example.